What is a Chemical Reaction?
When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. It consists of breaking existing bonds and forming new bonds by changing the position of electrons. These reactions are best explained using a chemical equation.
Chemical Reactions
Basically, a reactant (species involved in the reaction) is treated with a reagent (species provoking the change) to give a product (the transformed species). The reactant undergoes a chemical change to give the product. A typical chemical equation gives information about the starting molecule and its reaction conditions followed by the product. Sometimes it also gives information about the intermediate which is formed. Generally, the reaction intermediates can be ions (cations and anions) or free radicals.
A simple chemical reaction is given below; in which nitrogen and hydrogen react together to form a new species called ammonia.
N₂ + 3H₂ → 2NH3
The chemical reaction follows a sequence of steps that is consolidated as the mechanism of a reaction. This gives the idea of how the atoms and electrons are contributing towards the product formation. Apart from the reagents, other factors such as temperature and atmospheric pressure play an important role in a chemical reaction. To break bonds between the atoms thermal energy is required. Usually, when the temperature of a reaction is increased, the reaction rate also increases to provide this thermal energy.
Before reaching an equilibrium the two possible proceedings of a chemical reaction are forward or reverse. If they proceed in the forward direction they are known as spontaneous reactions whereas non-spontaneous reactions require a slight push towards equilibrium in the form of free energy.
The extension of chemical reactions to sub-atomic species existing is classified under nuclear reactions, radioactive decay, etc.
Chemical Equation
In chemistry, the reactions are typically represented using a chemical equation. In the equation, the reactants are given on the left hand side followed by an arrow with the reagents and reaction conditions mentioned above and below it that directs towards the products formed. The reactants are represented using symbols and formulas or structures with the coefficients indicating their stoichiometry.
C₂H6 + O₂ → CO₂ + H₂O
This equation is read as ‘ethane plus oxygen yields carbon dioxide and water.’
2C₂H6 + O₂ → 4CO₂ + 6H₂O
Whereas this reaction would be read with the coefficients like ‘two moles of ethane plus oxygen yields four moles of carbon dioxide and water.
Balancing a Chemical Equation
Based on the law of conservation of mass, in a chemical reaction, the quantity of the elements remains the same. Thus it is important for a chemical equation to be balanced on the reactant and the product side. The quantity of any particular element on the reactant side will not change on the product side.
The scalar number of a substance can be changed to balance the chemical equation either by inspecting the chemical equation or by solving a system of linear equations. One must use the smallest whole numbers to write a balanced equation. The molecule without a coefficient is understood to be 1.
A simple example is as follows:
A chemical equation - H₂ + O₂ → H₂O
A balanced chemical equation - 2H₂ + O₂ → 2H₂O
Here, the oxygen in the reactant and product were not in the same quantity and therefore a coefficient of 2 was added to the reactant side which makes it,
H₂ + O₂ → 2H₂O
But now the hydrogen quantity is more on the reactant side which can be balanced by adding the coefficient 2 in the reactant side.
2H₂ + O₂ → 2H₂O
Thereby a balanced chemical equation is achieved.
Similarly,
A chemical equation - HCl + Na → NaCl + H₂
A balanced chemical equation - 2 HCl + 2 Na → 2 NaCl + H₂
Initially the hydrogen on the product side was more in quantity than on the reactant side therefore it is balanced by adding a coefficient of 2 which gives -
2HCl + Na → NaCl + H₂
Now, the Cl quantity is imbalanced and therefore a coefficient to the sodium chloride is added.
2HCl + Na → 2NaCl + H₂
Finally, to balance Na, the coefficient 2 is added thereby attaining a balanced chemical equation.
2HCl + 2Na → 2NaCl + H₂
Types of Reaction

Synthesis
A complex molecule is generated by the combination of two or more molecules and is classified under the synthesis reactions. They are generally represented as -
X + Y → XY
Example: a combination of hydrogen gas and oxygen gas to generate water.
Decomposition
It is the opposite of synthesis because the combination of two molecules is broken down into separate molecules in a decomposition reaction. They are generally represented as
XY→ X + Y
Example: Water undergoes electrolysis to break down and give hydrogen gas and oxygen gas.
There is another reaction type called double decomposition. A double decomposition reaction occurs when two constituent reactants exchange positive and negative ions, resulting in the formation of two new products.
AB+CD → AD+CB
Single replacement
An element from the combined species is replaced by another single element present in the reaction mixture. They are generally represented as
XY + Z→ XZ + Y Example: the generation of magnesium hydroxide through the replacement of hydrogen in water by magnesium.
Double replacement
The generation of two entirely new species is when the anions and cations of two compounds interchange between themselves are classified under double replacement reaction. This is also referred to as double displacement reactions. They are generally represented as -
XY + AB→ XA + YB
Example: When HCl and NaOH react they form NaCl and water.
Other Classes of Chemical Reactions
Combustion
When energy is produced in the form of light or heat due to the reaction of a molecule with oxygen it is known as combustion. Combustion reactions generally involve oxygen and hydrocarbons.
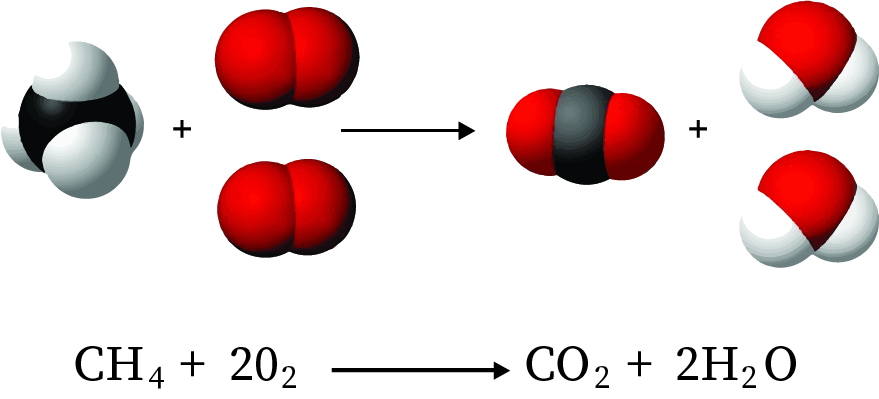
CH4(g) + 2 O₂ (g) → CO₂ (g) + 2 H₂O(g)
Oxidation
When the oxidation state of a molecule is increased during a reaction it is said to have undergone an oxidation reaction. Oxidation takes place in the presence of an oxidizing agent. For example, magnesium is oxidized to form magnesium oxide in the presence of oxygen.
Reduction
When the oxidation state of a molecule is decreased during a reaction it is said to have undergone a reduction reaction. Reduction takes place in the presence of a reducing agent. For example, ferric chloride in the presence of hydrogen undergoes reduction to form ferrous chloride.
2FeCl3 (aq) + H₂ (g) → 2FeCl₂ (aq) + 2HCl (aq)
Redox reaction
The transfer of electrons between two compounds due to which one compound is reduced and the other is oxidized simultaneously in the same reaction is known as a redox reaction, for example when hydrogen reacts with fluorine to form hydrogen fluoride. In this reaction, hydrogen undergoes oxidation and fluorine undergoes reduction simultaneously.
H₂ + F₂ → 2 HF
Context and Applications
Chemical reactions are the foundation of chemistry. Various synthesis and interactions can be studied using chemical reactions.
This topic is significant in the professional exams for both undergraduate and graduate courses, especially for Bachelors and Masters in Chemistry.
Want more help with your chemistry homework?
*Response times may vary by subject and question complexity. Median response time is 34 minutes for paid subscribers and may be longer for promotional offers.
Chemical Reactions and Equations Homework Questions from Fellow Students
Browse our recently answered Chemical Reactions and Equations homework questions.